Safety & dosing considerations for RHOPRESSA®
(netarsudil ophthalmic solution) 0.02%
Setting patient expectations about hyperemia
What to know about hyperemia
- ROCK inhibitors may cause vasodilation on the ocular surface1
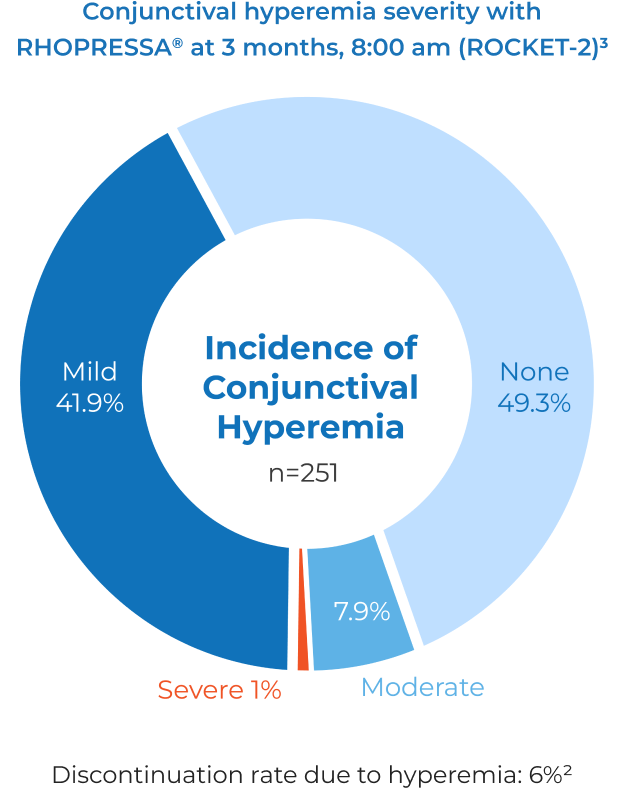
- 53% of patients experienced hyperemia in clinical studies; however, only 6% of patients discontinued for this reason2
What patients should know about hyperemia
- RHOPRESSA® causes blood vessels in the eye to dilate, which may result in visible redness1
- Redness alone does not necessarily indicate an allergic reaction. Instruct patients to inform you if they develop inflammation or irritation of the eye or eyelids
Most occurrences of hyperemia were rated by investigators as mild to moderate.3
See Important Safety Information about RHOPRESSA® on this page.
Help your patients get the most out of their RHOPRESSA® treatment
Help your patients get the most out of their RHOPRESSA® treatment with the MyAlcon Together Patient Support program
RHOPRESSA® should be taken once daily in the evening2
- The recommended dose for RHOPRESSA® is one drop in the affected eye(s) once daily in the evening2
- If you miss a dose of RHOPRESSA®, you should continue with your next dose the following evening2
- If more than 1 eye drop is being used, the drugs should be administered at least 5 minutes apart2
- Contact lenses should be removed prior to the administration of RHOPRESSA® and may be reinserted 15 minutes after administration2
- Avoid allowing the tip of the bottle to contact the eye, surrounding structures, fingers, or any other surface in order to minimize contamination of the solution. Serious damage to the eye and subsequent loss of vision may result from using contaminated solution
- Store RHOPRESSA® in the refrigerator (36 °F to 46 °F) until opened. After opening, RHOPRESSA® may be kept at 36 °F to 77 °F for up to 6 weeks. If after opening the product is kept refrigerated at 36 °F to 46 °F, then the product can be used until the expiration date stamped on the bottle2
ROCK=Rho kinase.
REFERENCES
1. Serle JB, Katz LJ, McLaurin E, et al. Two Phase 3 clinical trials comparing the safety and efficacy of netarsudil to timolol in patients with elevated intraocular pressure: Rho kinase elevated IOP treatment Trial 1 and 2 (ROCKET-1 and ROCKET-2). Am J Ophthalmol. 2018;186:116-127.
2. Rhopressa® (netarsudil ophthalmic solution) 0.02% Prescribing Information, Aerie Pharmaceuticals, Inc., Irvine, CA. 2019.
3. Alcon data on file, 2016.
IMPORTANT SAFETY INFORMATION
Warnings and Precautions
Epithelial Corneal Edema: Epithelial Corneal Edema, described as honeycomb or bollous, has been reported in some patients with pre-existing corneal stromal edema or following ocular procedures that could affect corneal endothelial function. Epithelial corneal edema typically resolves upon discontinuation of RHOPRESSA. Advise patients to notify their physician if they experience eye pain or decreased vision while using RHOPRESSA.
Bacterial Keratitis: There have been reports of bacterial keratitis associated with the use of multiple-dose containers of topical ophthalmic products. These containers had been inadvertently contaminated by patients who, in most cases, had a concurrent corneal disease or a disruption of the ocular epithelial surface.
INDICATIONS AND USAGE
Rhopressa® (netarsudil ophthalmic solution) 0.02% is indicated for the reduction of elevated intraocular pressure in patients with open-angle glaucoma or ocular hypertension.
Contact Lenses: Contact lenses should be removed prior to instillation of Rhopressa® and may be inserted 15 minutes following its administration.
Adverse Reactions
The most common ocular adverse reaction observed in controlled clinical studies with Rhopressa® dosed once daily was conjunctival hyperemia, reported in 53% of patients. Six percent of patients discontinued therapy due to conjunctival hyperemia. Other common (approximately 20%) adverse reactions were: corneal verticillata, instillation site pain, and conjunctival hemorrhage. Instillation site erythema, corneal staining, blurred vision, increased lacrimation, erythema of eyelid, and reduced visual acuity were reported in 5-10% of patients.
The corneal verticillata seen in Rhopressa®- treated patients were first noted at 4 weeks of daily dosing. This reaction did not result in any apparent visual functional changes. Most corneal verticillata resolved upon discontinuation of treatment.
Please click here for full prescribing information for Rhopressa®.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
DOSAGE AND ADMINISTRATION
The recommended dosage is one drop in the affected eye(s) once daily in the evening.
